Rybelsus 2026 and Semaglutide 2026: What Changed After 2025?
Why Interest Increased in 2026
Search interest in Rybelsus 2026 and semaglutide 2026 has surged for one simple reason: oral GLP-1 therapy is no longer a niche option. Patients want the benefits associated with semaglutide better glycemic control and meaningful weight reduction without injections, and clinicians want clear, evidence-based guidance on what’s new (and what isn’t).
This article is designed as a continuation of our existing Rybelsus coverage. It focuses on the post-2025 landscape: what changed in product positioning, what new research adds to clinical decision-making, and what patients should pay attention to in 2026. If you’re starting from zero and want the full background on how semaglutide works, the dosing logic across brands, and key safety considerations, begin with our evidence-based overview of semaglutide here: Semaglutide overview (2025 guide for U.S. patients.
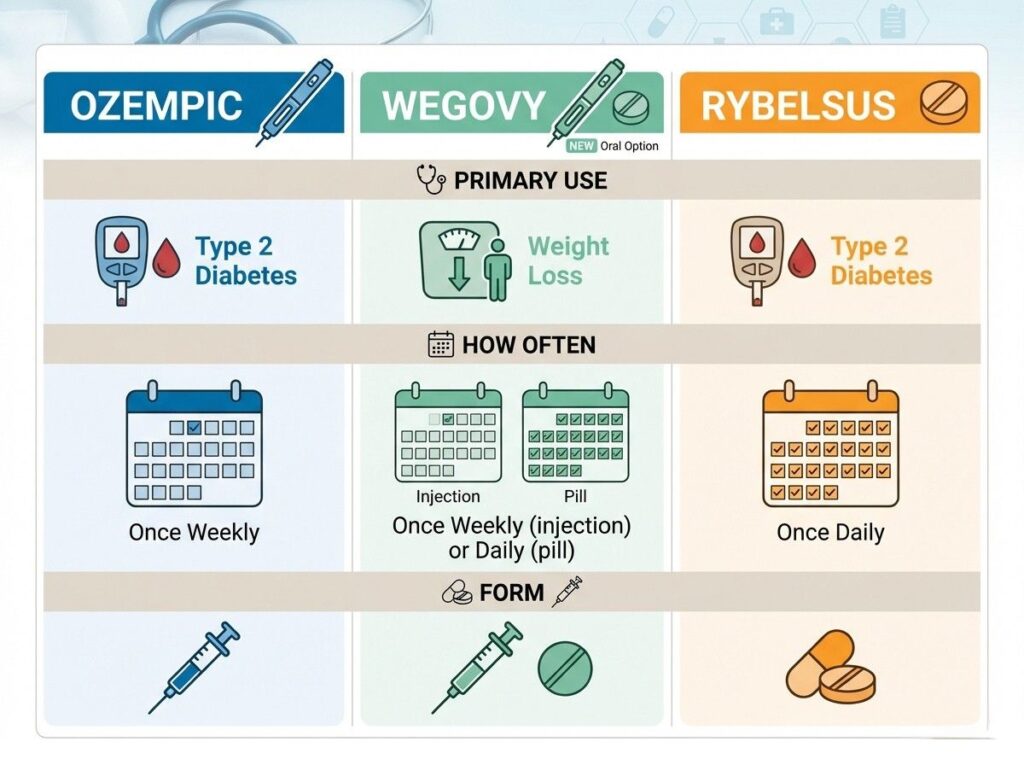
A quick clarification that matters for accuracy: Rybelsus remains the established brand name for oral semaglutide in major markets, while Ozempic refers to injectable semaglutide for type 2 diabetes and Wegovy to injectable semaglutide for chronic weight management. You may see the phrase “oral Ozempic” used informally online, but it is not a label you should rely on when discussing approved product names or prescribing information.
What Was Rybelsus Like in 2025? The Baseline for Comparison
To understand the “what changed after 2025” question, you need a clean baseline. In 2025, Rybelsus was widely discussed for two overlapping reasons: its established role in type 2 diabetes care and the growing attention to semaglutide’s weight-related outcomes across the GLP-1 class.
Rybelsus in clinical practice (2025 snapshot)
In 2025, Rybelsus was positioned primarily as an oral GLP-1 receptor agonist for adults with type 2 diabetes. It was commonly discussed alongside injectables, especially in patients who wanted GLP-1 benefits but preferred tablets. Our clinical update from that period (focused specifically on diabetes use and how it fits into treatment plans) is here: Rybelsus for type 2 diabetes updated overview.
A defining feature of Rybelsus has always been how it must be taken to work as intended. Because oral semaglutide absorption is sensitive to timing and stomach contents, real-world effectiveness depends heavily on administration adherence.
Key 2025 baseline point: Rybelsus used a strict dosing routine and established strengths; patients were often reminded that “how you take it” can matter as much as “what dose you take.”
Where weight loss fit into the 2025 conversation
Even when the primary indication was diabetes, weight outcomes became an increasingly common reason patients asked about Rybelsus in 2025. Interest was fueled by the broader semaglutide data ecosystem and by patient preference for oral options. If your site audience is specifically searching for “Rybelsus for weight loss” (and how oral semaglutide compares to other approaches), this analysis provides the context people were using in 2025: Rybelsus for weight loss how oral semaglutide is changing obesity treatment.
What “changed in 2025” without overclaiming
Many online summaries of 2025–2026 changes mix confirmed facts with speculation. For a regulatory-safe, evidence-first article, it’s important to describe 2025 like this:
- Confirmed baseline: Rybelsus was the recognized oral semaglutide product, used primarily for type 2 diabetes, with well-known administration requirements and established dosing strengths per local labeling.
- Active research direction: higher-exposure oral semaglutide regimens were being studied in obesity-focused programs, helping set expectations about where oral semaglutide might go next.
- Market reality: public interest in GLP-1s increased sharply, driving more questions about oral options, switches between brands, and long-term outcomes.
That framing gives you a clean “before” picture-without claiming dose changes or rebrands that may not be universally confirmed across regions.
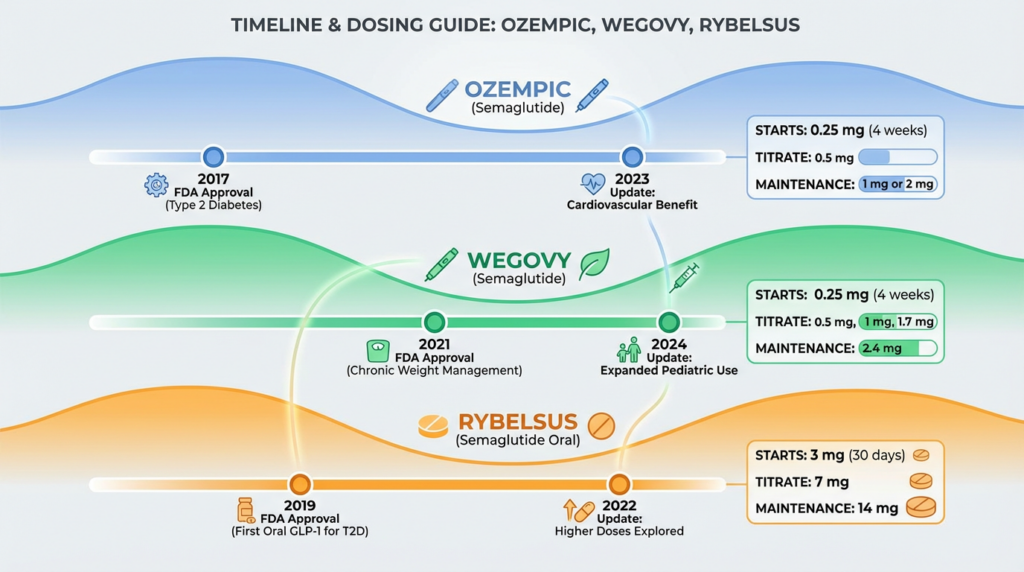
What Changed in 2026? Positioning, Evidence and Patient Expectations
Moving into 2026, the most important shift is not a dramatic rebranding or a universal dose replacement. The real change is how oral semaglutide is positioned within cardiometabolic care and how strongly it is supported by outcome data.
First, it is essential to clarify product identity. Rybelsus continues to be the established brand name for oral semaglutide in the United States and in most major regulated markets. Ozempic remains the injectable form for type 2 diabetes, and Wegovy remains the injectable form approved for chronic weight management. Informal online references to an “Ozempic pill” reflect search behavior, not an officially distinct FDA approved product name.
Dosing and formulation reality in 2026
As of the most recent confirmed regulatory labeling in the United States, Rybelsus is approved in 3 mg, 7 mg, and 14 mg tablet strengths. There has been ongoing research into higher exposure oral semaglutide regimens for obesity, but those research programs do not automatically translate into globally approved retail doses.
For readers searching phrases such as “new Rybelsus doses 2026” or “Rybelsus formula update,” the accurate summary is this:
- The core approved strengths for type 2 diabetes remain consistent with official labeling
- Administration instructions remain strict due to absorption requirements
- Research programs continue to explore expanded metabolic applications
Because oral semaglutide absorption depends on gastric conditions, the administration rules still require taking the tablet on an empty stomach with a small amount of water and waiting before eating or taking other medications. This has not changed in 2026 and remains central to treatment success.
If your audience is comparing oral and injectable options in 2026, this side by side clinical comparison provides structured context: Ozempic vs Rybelsus comparison guide.
Cardiovascular Evidence Strengthened the 2026 Narrative
The most meaningful evolution in 2026 is the reinforcement of semaglutide’s cardiovascular benefit profile.
The SOUL trial evaluated oral semaglutide in patients with type 2 diabetes who were at high cardiovascular risk. The results demonstrated a statistically significant reduction in major adverse cardiovascular events. This aligns oral semaglutide more closely with the cardiovascular benefit profile previously demonstrated in injectable semaglutide studies.
In parallel, the SELECT trial, which studied injectable semaglutide in people with overweight or obesity without diabetes, confirmed reduced cardiovascular events in that population. While SELECT used an injectable formulation, the broader implication is that GLP 1 receptor agonism itself plays a role in cardiometabolic risk reduction.
For an expanded perspective on how GLP 1 therapies fit into modern obesity pharmacotherapy, see: Obesity as a chronic disease pharmacological treatment approaches.
OASIS and the Expanding Obesity Conversation
The OASIS clinical development program studied higher exposure oral semaglutide regimens in people with overweight or obesity. Certain trial arms reported average weight reduction in the range of approximately 13 to 15 percent.
It is important to separate clinical trial outcomes from regulatory approval status. Not every studied dose becomes an approved commercial product in every country. However, the data strengthen the scientific rationale for oral semaglutide as a potential obesity treatment pathway where authorized.
Safety and Legal Landscape in 2026
The safety profile of semaglutide in 2026 remains consistent with prior years. The most common adverse effects continue to involve gastrointestinal symptoms such as nausea, vomiting, and diarrhea, particularly during dose escalation.
There has been increased public discussion and litigation involving GLP 1 medications as a class. However, regulatory authorities continue to consider semaglutide’s benefit risk profile favorable when used according to approved labeling and under medical supervision.
The key clinical principle remains unchanged. Appropriate patient selection, adherence to dosing instructions, and physician monitoring are central to minimizing risk and optimizing outcomes.
Rybelsus 2026 vs Alternatives: How to Decide
By 2026, patients are no longer asking whether GLP 1 therapy works. The more common question is which formulation fits their medical profile and lifestyle best.
When comparing Rybelsus with injectable semaglutide, the difference is primarily route of administration and exposure profile. Injectable options may achieve higher maximal exposure at certain approved doses, but oral semaglutide offers a needle free alternative that some patients find easier to integrate into daily routines. The choice often comes down to clinical goals, tolerance, adherence expectations, and physician guidance.
For a structured breakdown of oral versus injectable semaglutide in diabetes and weight management, see: Ozempic vs Rybelsus clinical comparison.
Older weight loss medications such as orlistat work through different mechanisms and typically produce more modest average weight reduction compared to GLP 1 receptor agonists. If your audience is actively comparing oral semaglutide with traditional fat absorption blockers, this detailed guide provides context: Rybelsus and Xenical comparison.
In 2026, Rybelsus may be particularly relevant for:
- Adults with type 2 diabetes seeking glycemic control with cardiovascular outcome data
- Patients who prefer tablets over injections
- Individuals who can reliably follow the specific administration timing requirements
Final treatment decisions should always involve individualized risk assessment by a licensed healthcare professional.
Rybelsus in 2026 and What Patients Should Know
The most accurate way to describe Rybelsus in 2026 is not as a radically changed product, but as a therapy supported by stronger long term outcome data and broader cardiometabolic context.
Rybelsus remains the established semaglutide brand for type 2 diabetes in the United States and other regulated markets. Cardiovascular outcome evidence from recent trials strengthens confidence in its role within modern diabetes management. Ongoing obesity focused research continues to expand the scientific conversation around oral semaglutide’s potential applications.
For patients evaluating therapy options, the 2026 landscape offers clearer data, not hype. The fundamentals remain the same: proper dosing, realistic expectations, and physician guided monitoring.
If you want detailed information about currently approved doses, availability, and pricing, visit: Buy Rybelsus dosage and pricing information.
Medical Disclaimer
This article is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional regarding any medical condition or treatment.
Author:
Dr. Cody R. Christensen
F.A.Q: Rybelsus in 2026
Has Rybelsus changed its approved doses in 2026?
In the United States, Rybelsus remains approved in 3 mg, 7 mg, and 14 mg tablet strengths according to official labeling. Any additional regimens discussed online may refer to clinical trials rather than approved retail products.
Is there an official product called Ozempic pill?
No separate FDA approved product carries that exact name. The oral semaglutide formulation is marketed as Rybelsus, while Ozempic refers to the injectable version.
Does oral semaglutide reduce cardiovascular risk?
The SOUL trial demonstrated reduced major adverse cardiovascular events in high risk patients with type 2 diabetes. This supports the broader cardiometabolic benefit profile of semaglutide therapy.
Can Rybelsus be used for weight loss in 2026?
Weight reduction has been observed in clinical studies of oral semaglutide, including obesity focused research programs. Regulatory approval for weight management indications depends on the specific country and labeling.
Has the safety profile changed recently?
The safety profile remains consistent with previous years, with gastrointestinal symptoms being the most common side effects. Appropriate medical supervision remains essential for safe use.
Sources
- U.S. Food and Drug Administration (FDA) – Rybelsus Prescribing Information & Approval History
- European Medicines Agency (EMA) – Rybelsus (semaglutide) EPAR Product Information
- The New England Journal of Medicine – Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes (SELECT Trial)
- American Diabetes Association – Standards of Care in Diabetes 2025 (Cardiovascular and GLP-1 Guidance)
